Introduction¶
Spectroscopies are among the most widely used techniques to study the physical and chemical properties of materials. They are now extensively present in many fields of science such as physics, chemistry or biology. The term “spectroscopy” which was still characterizing the study of light by means of a prism in the 19th century, has now, since the advent of quantum theory, a much wider meaning. Indeed, according to the 15th edition of The New Encyclopaedia Britannica, “spectroscopy is the study of the absorption and emission of light and other radiation, as related to the wavelength of the radiation”. If we add scattering to the two previous physical processes, the quantum nature of particles makes this definition cover most types of experiment that can be performed nowadays. Such a typical experiment is sketched below. It should be noted that the incoming and outgoing particles are not necessarily the same. When they are of an identical nature, the corresponding technique can be performed either in the reflection or in the transmission mode.
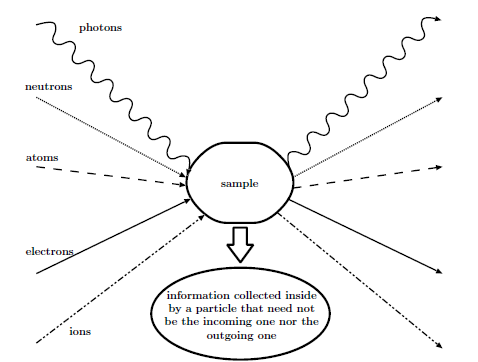
Typical spectroscopic experiment¶
The idea behind these spectroscopies is that information about the sample can be obtained from the analysis of the outgoing particles. Depending on the type of spectroscopy, this information can be related to the crystallographic structure, to the electronic structure or to the magnetic structure of the sample. Or it can be about a reaction that takes place within the sample. With this in mind, the type and energy of the detected particles will directly determine the region of the sample from which this information can be traced back, and therefore the sensitivity of the technique to the bulk or the surface. For instance, low-energy electrons or ions will not travel much more than ten interatomic distances in a solid while electromagnetic radiations will be able to emerge from much deeper layers. In diffraction techniques using significantly penetrating particles, surface sensitivity can nevertheless be achieved with grazing incidence angles. Note also that the counterpart to detecting particles with a small mean free path is the necessity to work under ultra high vacuum conditions so that the outgoing particles can effectively reach the detector. The figure below gives the variation of the electron mean free path \(\lambda_e\) in solids as a function of the electron kinetic energy. We see clearly here that in the range 10–1000 eV, electrons coming from within a sample do not originate from much deeper than 5 to 20 Angströms. As a consequence, spectroscopies detecting electrons in this energy range will be essentially sensitive to the surface structure as the particle detected will carry information from the very topmost layers.
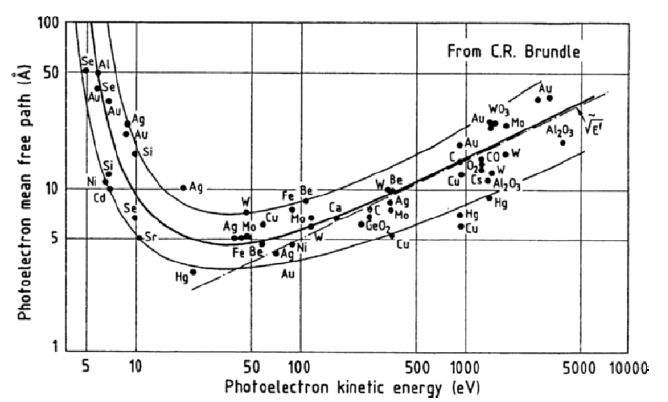
The electron mean free path as a function of the energy¶
Although, as we have just seen, many techniques can provide information on a sample and its surface (if any), we will mainly restrict ourselves here to some of them specifically related to synchrotron radiation: x-ray spectroscopies. X-ray spectroscopies are characterized by the fact that the incoming beam is composed of photons in the range of about 100 eV to 10 keV. Higher energies, in the \(\gamma\)-ray region, will not be considered here as in this latter range photons will be scattered significantly not only by the electrons but also by the nuclei [2] giving rise to entirely different spectroscopies such as Mössbauer spectroscopy. The next, taken from [3], gives a sketch of the electromagnetic spectrum with some common photon sources and the spectroscopies corresponding to the various energy ranges.
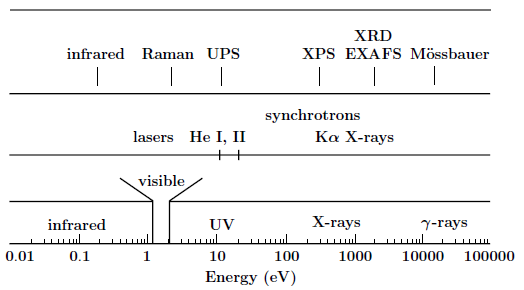
The electromagnetic spectrum, along with the common photon sources and some spectroscopies based on photons.¶
See also
This introduction is taken from:
- X-ray and Electron Spectroscopies: An Introduction
Didier Sébilleau, Lect. Notes Phys. 697, p15–57 (2006) [doi]
For a more complete theoretical background about multiple scattering, see (and references herein):
- Multiple-scattering approach with complex potential in the interpretation of electron and photon spectroscopies
D. Sebilleau, R. Gunnella, Z-Y Wu, S Di Matteo and C. R. Natoli, J. Phys.: Condens. Matter 18, R175-228 (2006) [doi]
For a description of the MsSpec code package, see:
- MsSpec-1.0: A multiple scattering package for electron spectroscopies in material science
D. Sébilleau, C. R. Natoli, G. M.Gavaza, H. Zhao, F. Da Pieve and K. Hatada, Comput. Phys. Commun., 182 (12), p2567-2579 (2011) [doi]